Good News | BioDetect‘s Six respiratory pathogen nucleic acid test kit(Quantitative Real-time polymerase chain reaction melting curve method) were approved for the Chinese domestic market!
On Jan13, 2025, BioDetect’s received registration approval from the National Medical Products Administration for its Six respiratory pathogen nucleic acid test kit(Quantitative Real-time polymerase chain reaction melting curve method), with the registration certificate number: Guo Xie Zhu Zhun 20253400065.
Six respiratory pathogen nucleic acid test kit(Quantitative Real-time polymerase chain reaction melting curve method)
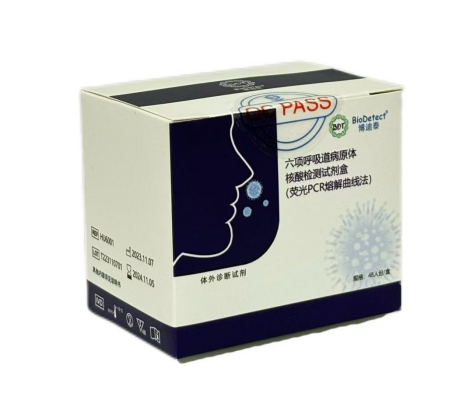
【Intended Use】:
This kit is used for qualitative detection of novel coronavirus 2019 nCoV Influenza A virus, influenza B virus, respiratory syncytial virus, adenovirus, and Mycoplasma pneumoniae Nucleic acid. This product cannot distinguish specific types of pathogens
【Packaging Specifications】:
48 test/box
About Us
Biodetect (Xiamen) Biotechnology Co., Ltd. was founded by the overseas doctor team on July 04, 2017, specializing in the development and production of in vitro diagnostic reagents, and was selected as the leading entrepreneurial talent project of Xiamen "Double Hundred Plan" in 2017. The company is headquartered in Xiamen Biopharmaceutic Industrial Park, a national incubation base. The core technical team brings together a number of postdocs, doctors and overseas returnees. Academicians of the Chinese Academy of Sciences and experts of the "Thousand Talents Plan" serve as technical advisers.



