Good News | BioDetect‘s Rapid FluA / B combined Antigen Detection Kit (Colloidal gold) were approved for the Chinese domestic market!
On September 25, 2024, BioDetect’s received registration approval from the National Medical Products Administration for its Rapid FluA / B combined Antigen Detection Kit (Colloidal gold), with the registration certificate number: Guo Xie Zhu Zhun 20243401885.
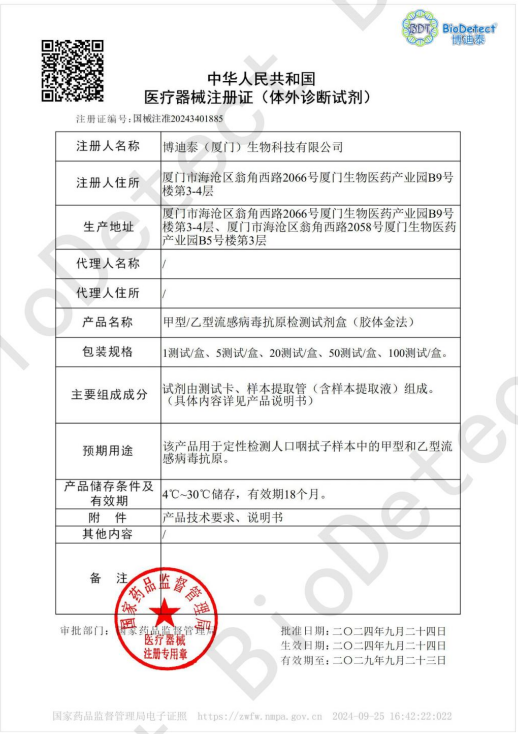
FluA / B combined Antigen Detection Kit (Colloidal gold)

【Intended Use】:
This product is designed for the qualitative detection of Influenza A and B virus antigens in oropharyngeal swab samples from individuals. The results obtained from this kit are intended for clinical reference only and should not be used as the sole criterion for clinical diagnosis. It is recommended to combine patient clinical manifestations with other laboratory tests for a comprehensive analysis of the condition. Influenza A and B are both respiratory infections caused by influenza viruses. Influenza A virus is an RNA virus characterized by two proteins on its surface: hemagglutinin (H) and neuraminidase (N), which combine in various ways to form different subtypes, such as H1N1, H3N2, H5N1, etc. Influenza A virus undergoes frequent mutations, while Influenza B virus has lower variability compared to Influenza A, but still exhibits some degree of mutation. Symptoms of Influenza A infection typically include high fever, headache, muscle aches, cough, sore throat, nasal congestion, runny nose, and fatigue. It can lead to severe complications like pneumonia, heart disease, myasthenia gravis, encephalitis, etc. Symptoms of Influenza B infection are generally similar to those of Influenza A but usually do not result in severe complications. This product employs colloidal gold immunochromatographic technology and is suitable for auxiliary diagnosis of Influenza A and B virus infections. This product should not be used alone as a basis for treatment or diagnosis.
【Packaging Specifications】:
1 test/box, 5 tests/box, 20 tests/box, 50 tests/box, 100 tests/box.
【Storage Conditions and Shelf Life】:
Store at 4℃~30℃, shelf life is 18 months.
After opening the aluminum foil bag, it is advised to use the test card within 1 hour at 4℃~30℃, humidity <60%. Once the sample extraction liquid is unsealed, please use it as soon as possible within 1 hour.
See the outer packaging for the production date and expiration date.
About Us

Biodetect (Xiamen) Biotechnology Co., Ltd. was founded by the overseas doctor team on July 04, 2017, specializing in the development and production of in vitro diagnostic reagents, and was selected as the leading entrepreneurial talent project of Xiamen "Double Hundred Plan" in 2017. The company is headquartered in Xiamen Biopharmaceutic Industrial Park, a national incubation base. The core technical team brings together a number of postdocs, doctors and overseas returnees. Academicians of the Chinese Academy of Sciences and experts of the "Thousand Talents Plan" serve as technical advisers.



