Heavy! ! ! Our company is listed in the list of key medical material production enterprises in Fujian Province in 2022 (the second batch)
A few days ago, the Fujian Provincial Department of Industry and Information Technology announced a list of key medical material manufacturers with a certain scale, production qualifications, and continuous production for reference in emergency procurement. Our company ranks among them with excellent product quality and complete medical qualifications. Two products, "pharyngeal swab" and "disposable virus sampling tube" entered the list respectively.

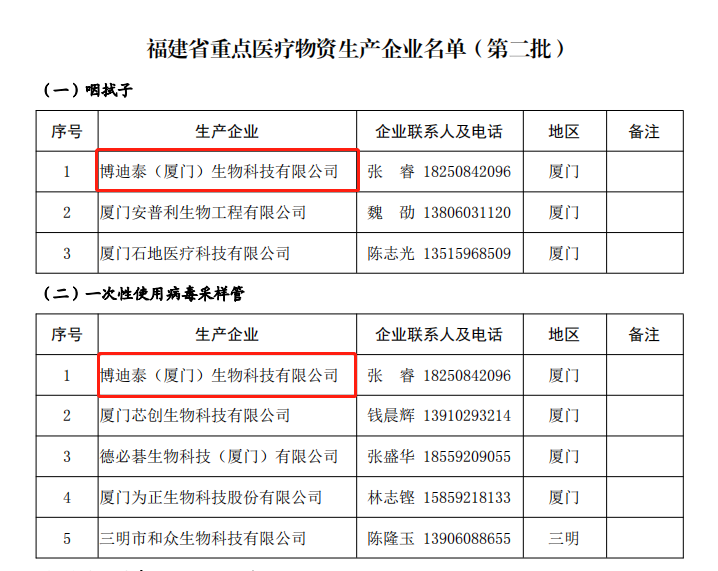
The notice requires that the corresponding local industry and information technology departments of enterprises should strengthen tracking services, ensure the raw materials, production equipment, employees, logistics, water, electricity, oil and gas and other production factors of enterprises in the catalog, coordinate and solve difficult problems of enterprises, and ensure efficient production. It means that the qualification of our company's epidemic prevention materials has been affirmed by relevant departments, and the production capacity of the enterprise has also been fully supported. Ensure the effective guarantee of the supply of epidemic prevention materials during the epidemic, facilitate the timely and stable supply of epidemic prevention materials in high-risk areas of the epidemic in Fujian Province, and quickly win the battle against epidemic prevention and control.
The following are selected products
1. Disposable virus sampling tube
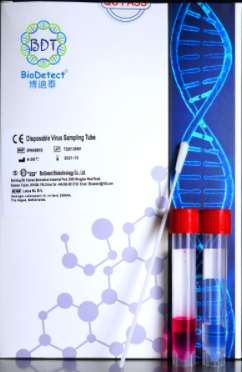
This product is a disposable virus sampling tube, which is used for sampling and transporting respiratory specimens such as novel coronavirus, influenza virus (common influenza, highly pathogenic avian influenza, H1N1 influenza virus, etc.), SARS, etc. It is suitable for disease control departments and clinical departments to monitor and sample infectious pathogenic microorganisms. It is used to transport nasopharyngeal swab samples or tissue samples from specific parts from the sampling site to the testing laboratory for subsequent extraction and testing.
This product contains virus lysate and virus nucleic acid preservation solution, which can quickly disinfect the virus and prevent the secondary transmission of the virus to medical staff during the sampling process. At the same time, the virus nucleic acid preservation solution can stably preserve the sample nucleic acid, ensuring the accuracy of virus nucleic acid detection accuracy.
2. Throat swab
This product is a single-use sampler for sampling respiratory specimens such as novel coronavirus, influenza virus, and SARS influenza virus.


As a biomedical high-tech company, BioDetect is committed to making contributions to the medical industry and human health. We insist on dedicating value to human health and well-being with unremitting technological pursuit and continuous innovation ability. We are committed to the R&D and production of new molecular diagnostic reagents. We have a core technology platform with independent intellectual property rights, focusing on innovative gene editing constant temperature nucleic acid amplification technology for rapid gene diagnosis.



